More than 400,000 bottles of non-prescription pain medication offered throughout the United States have actually been recalled due to the fact that the bottles are not child-resistant, according to notifications published on the U.S. Customer Product Safety Commission’s (CPSC) site.
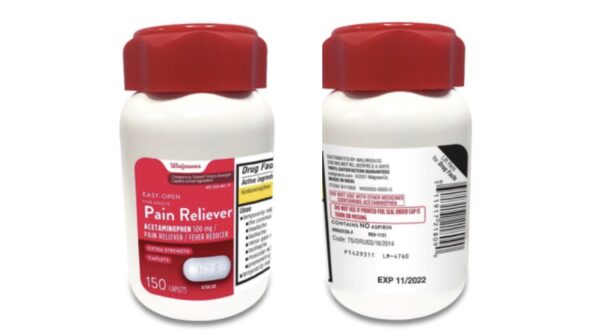
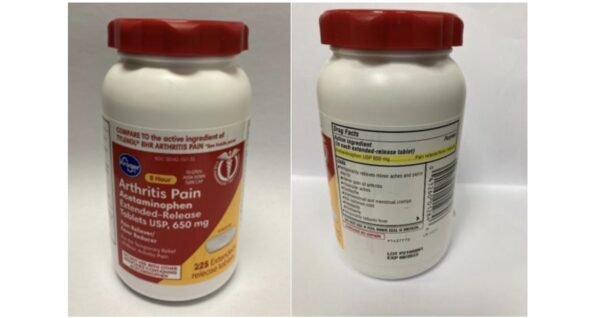
Aurohealth has reportedly recalled about 137,300 units of Walgreens brand acetaminophen and 25,660 units of Kroger brand arthritis pain acetaminophen.
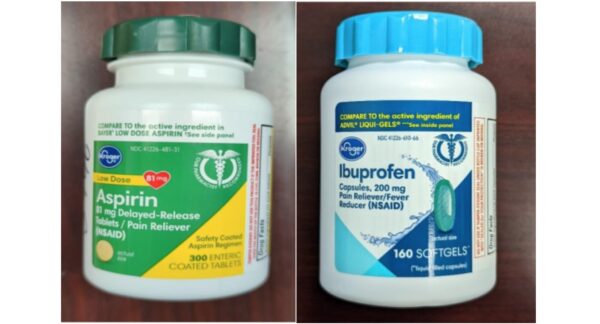
Around the same time, approximately 209,430 units of Kroger brand aspirin and ibuprofen were recalled by Time-Cap Lab, as well as an additional 34,660 units of Kroger brand acetaminophen recalled by Sun Pharma.

All the remembered products are bottles that are not child-resistant, presenting a threat of poisoning if kids handle to open the bottles and swallow the contents. The Poison Prevention Packaging Act (PPPA), signed into law in 1970, needs child-resistant product packaging.
“The packaging required by the PPPA must be designed or constructed to be significantly difficult for children under five years of age to open within a reasonable time, and not difficult for normal adults to use properly,” the CPSC states.
Individuals who actually have these recalled products are recommended to store them in a safe place “out of reach and sight of children.”
For people who bought the Walgreens brand name acetaminophen, they are recommended to get in touch with Aurohealth for info on how to return the items to their nearby Walgreens shop for a complete refund.
For those who bought the other items, which are Kroger brand name, they are encouraged to call Kroger on how to appropriately dispose of the item and get a complete refund.
Product recalls have struck a 10-year high in the United States this year, with more than 900 million systems of items remembered in a variety of markets throughout the very first quarter of 2022, according to a report distributed by insurance company Sedgwick in late May.
H/T The Epoch Times









